Effect Of Germicidal UV On Plastic Materials
Much of the effect of sunlight on materials has been attributed to the UV component (IESNA 2000), UV can fade some wall paints, wallpapers, and drapery fabrics (GE 1950). Some materials may have high UV reflectivity, like aluminum, or have high transmissivity, like quartz which absorbs very little UV. The absorption of UV by itself, is not necessarily an indicator that UV damage may occur, since it is the photochemistry which determines material effects. The total absorption is, therefore, an indicator of the potential for photodegradation in materials, while reflectivity can indicate protective effects.
UV photons energy vs. chemical bonds
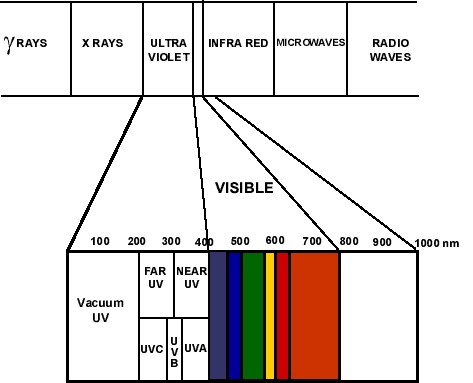
When polymers are exposed to ultraviolet light, i.e. 100–400 nm wavelength, the photons energy exceeds the bond energy of the carbon bonds in the polymer or else exceeds the activation energy of chemical reaction (Moreau and Viswanathan 1976). The depth to which ultraviolet light penetrates the polymer creates a region of absorption where photochemical reaction may take place, and where photodegradation may occur. Since UV transmissivity tends to be very low for most materials, even at millimeter thicknesses, most of the photodegradation will occur on the immediate surface of a material, to a depth of typically less than 0.01 to 0.1 millimeter. For most common polymers the depth of UV penetration is typically about 0.025 mm to 0.050 mm i.e. 25 to 50 microns.
In the photodeterioration of paints, varnishes, and textiles, the quantum yield is several order of magnitudes less than unity (Feller 1994). For the bleaching of certain dyes the quantum yield has been reported to be about 0.002, meaning a thousand photons must be absorbed before two molecules are bleached. Quantum yields as low as 0.0001 (10,000 photons per molecule) have been reported for most plastics. High quality pure plastics are relatively resistant to UV but impurities and residual solvents in low-grade plastics are mainly responsible for their quick photodegradation.
Yellowing of polymers from ultraviolet exposure tends to be concentrated on the immediate surface. Surface yellowing tends to block UV and protects the inner plastic. The fading of pigments and dyes can be evaluated in terms of the loss in concentration over time (Feller 1994). The depth of discoloration is reduced by the presence of color pigments. As the concentration of pigments increases, the depth of discoloration or fading also decreases.
Plastic properties and protection against degradation
There are as many as thirteen different properties of plastics which can be used as indicators of photodegradation, including coloration, tensile strength, elongation, hardness, degree of polymerization, infrared absorbance, etc. Experimental data indicates the response of most of these properties to extended ultraviolet exposure results in data that can be effectively modeled with exponential decay curves of one or more orders.
Materials that would darken to UV after exposure create a thin UV-proof film on the surfaces of polymers like PVC. This would enable them to develop resistance to further UV exposure (Owen 1976).
The photochemical degradation of materials is a dose-dependent function that depends only on the quantum yield and the molar absorption coefficient at the irradiation wavelength (Bolton and Stefan 2002). It describes the susceptibility of a material to degrade under UV exposure. Associated with this there would be some limiting distance, a film thickness or penetration depth, to which UV would penetrate.
Based on several decades of use, experience has shown that within a few exceptions, the UV induced damages tend to remain superficial and do not generally affect the structural or mechanical integrity of thick plastic components. For critical components such as exposed electrical wire direct insulation coating, it is recommended to cover the wires with aluminum tape or run the wires inside protective metallic rigid or flex conduits according to good practice and general electrical codes prescriptions. Rubbers in general such as motor belts and conduits used in the HVAC industry have proven to stand germicidal UV very well over the last 20 years of cumulated field experience.
Screens of many electronic devices can be affected by UV degradation due to the grade of plastic used and the very thin film generally used. For such devices, protection is easily achieved by installing with a simple glass window of 3 mm thickness over the screen. Transmittivity of common amorphous glass approaches zero for below 370 nm wavelength.
References
IESNA. 2000. Lighting Handbook: Reference & Application IESNA HB-9-2000. New York: Illumination Engineering Society of North America.
GE. 1950. Germicidal Lamps and Applications. USA: General Electric. Report nr SMA TAB: VIII-B.
Moreau W, Viswanathan N. 1976. Applications of Radiation Sensitive Polymer Systems. In: Labana SS, editor. Ultraviolet Light Induced Reactions in Polymers. Washington, DC: Ameri- can Chemical Society, pp. 107–134.
Feller RL. 1994. Accelerated Aging: Photochemical and Thermal Aspects. Institute TGC, editor.
Ann Arbor, MI: Edwards Bros.
Bolton J, Stefan M. 2002. Fundamental photochemical approach to the concepts of fluence (UV Dose) and electrical energy efficiency in photochemical degradation reactions. Res Chem Intermed 28(7–8):857–870.
Owen ED. 1976. Photodegradation of Polyvinyl chloride. In: Labana SS, editor. Ultraviolet Light Induced Reactions in Polymers. Washington, DC: American Chemical Society, pp. 208–219.
HEADQUATERS
PG35, Damansara Perdana Shopping Centre, Jalan PJU 8/1, 47820, Petaling Jaya, Selangor, Malaysia
TEL : 0377332757 EMAIL:info@ansrcare.com
BRANCH
Lot 3484 B, Tingkat Bawah Wisma Seri Panji Alam Jalan Panji Alam 21100 Kuala Terengganu, Terengganu Darul Iman.
TEL:096229270 EMAIL:info@ansrcare.com
ANSRCARE.COM © 2018, All Rights Reserved. Web Design By BG Design